Implementing pharmaceutical reverse logistics comes with a set of key challenges. From ensuring regulatory compliance and maintaining product integrity to managing costs and stakeholder collaboration, these challenges span areas such as traceability, technology adoption, and global supply chain complexities. In this article, we will delve into the critical challenges in implementing pharmaceutical reverse logistics and explore potential solutions to overcome them.
1. Regulatory compliance:
Ensuring compliance with complex regulations governing the reverse logistics of pharmaceutical products can be challenging. These regulations vary across different jurisdictions and require careful adherence to avoid legal and financial repercussions.
2. Product integrity in pharmaceutical reverse logistics:
Maintaining the integrity of pharmaceutical products during the reverse logistics process is crucial. Proper handling, storage, and transportation are essential to prevent contamination, damage, or expiration of the products, which could pose risks to patient safety.
3. Traceability and serialization:
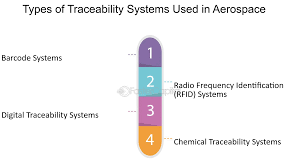
Tracking pharmaceutical products throughout the reverse logistics chain is essential to ensure transparency and prevent counterfeiting. Implementing robust traceability systems and serialization methods can be a significant challenge due to the complexity and scale of the pharmaceutical supply chain.
4. Disposal of hazardous materials:
Proper disposal of pharmaceutical waste, including expired or damaged products, is a critical challenge. Pharmaceutical waste often contains hazardous substances that require special handling and disposal procedures to protect the environment and public health.
5. Cost management in pharmaceutical reverse logistics :
Implementing efficient reverse logistics processes can be costly, primarily due to the need for specialized infrastructure, transportation, and personnel. Balancing cost-effectiveness while meeting regulatory requirements and ensuring product integrity is a key challenge for pharmaceutical companies.

6. Stakeholder collaboration:
Effective implementation of pharmaceutical reverse logistics requires collaboration among various stakeholders, including manufacturers, distributors, healthcare providers, and regulatory authorities. Ensuring smooth coordination and information sharing among these stakeholders can be a complex challenge.
7. Technology and data management:
Leveraging technology and data management systems is crucial for optimizing reverse logistics operations in the pharmaceutical industry. However, adopting and integrating advanced technologies, such as track-and-trace systems or data analytics, can present implementation challenges, especially for smaller companies with limited resources.
8. Patient privacy and data security:

Reverse logistics processes involve handling and managing sensitive patient information, such as product return details or patient-specific data. Ensuring compliance with privacy regulations and maintaining robust data security measures is a significant challenge to protect patient privacy and prevent data breaches.
9. Education and awareness in pharmaceutical reverse logistics:
Creating awareness and educating stakeholders about the importance of implementing effective reverse logistics practices in the pharmaceutical industry is a persistent challenge. Many stakeholders may lack knowledge or understanding of the benefits and best practices associated with reverse logistics, making it difficult to drive widespread adoption.
10. Global supply chain complexities:
The pharmaceutical industry operates on a global scale, with complex supply chains spanning multiple countries. Coordinating reverse logistics activities across different regions, each with its unique regulations, infrastructure, and cultural factors, presents significant challenges in implementing standardized practices and ensuring consistency.
Conclusion
Implementing pharmaceutical reverse logistics poses several key challenges that need to be addressed by industry stakeholders. These challenges encompass regulatory compliance, product integrity, traceability, hazardous waste disposal, cost management, stakeholder collaboration, technology adoption, patient privacy, education and awareness, and global supply chain complexities. Overcoming these challenges requires proactive measures, such as robust compliance strategies, efficient handling and storage practices, advanced traceability systems, proper waste disposal procedures, cost-effective solutions, effective collaboration, technological advancements, data security measures, awareness campaigns, and streamlined coordination across regions. By addressing these challenges, the pharmaceutical industry can enhance the effectiveness and efficiency of reverse logistics processes, ensuring the safe and sustainable handling of pharmaceutical products throughout their lifecycle.
Check out our previous blog post by clicking Here

